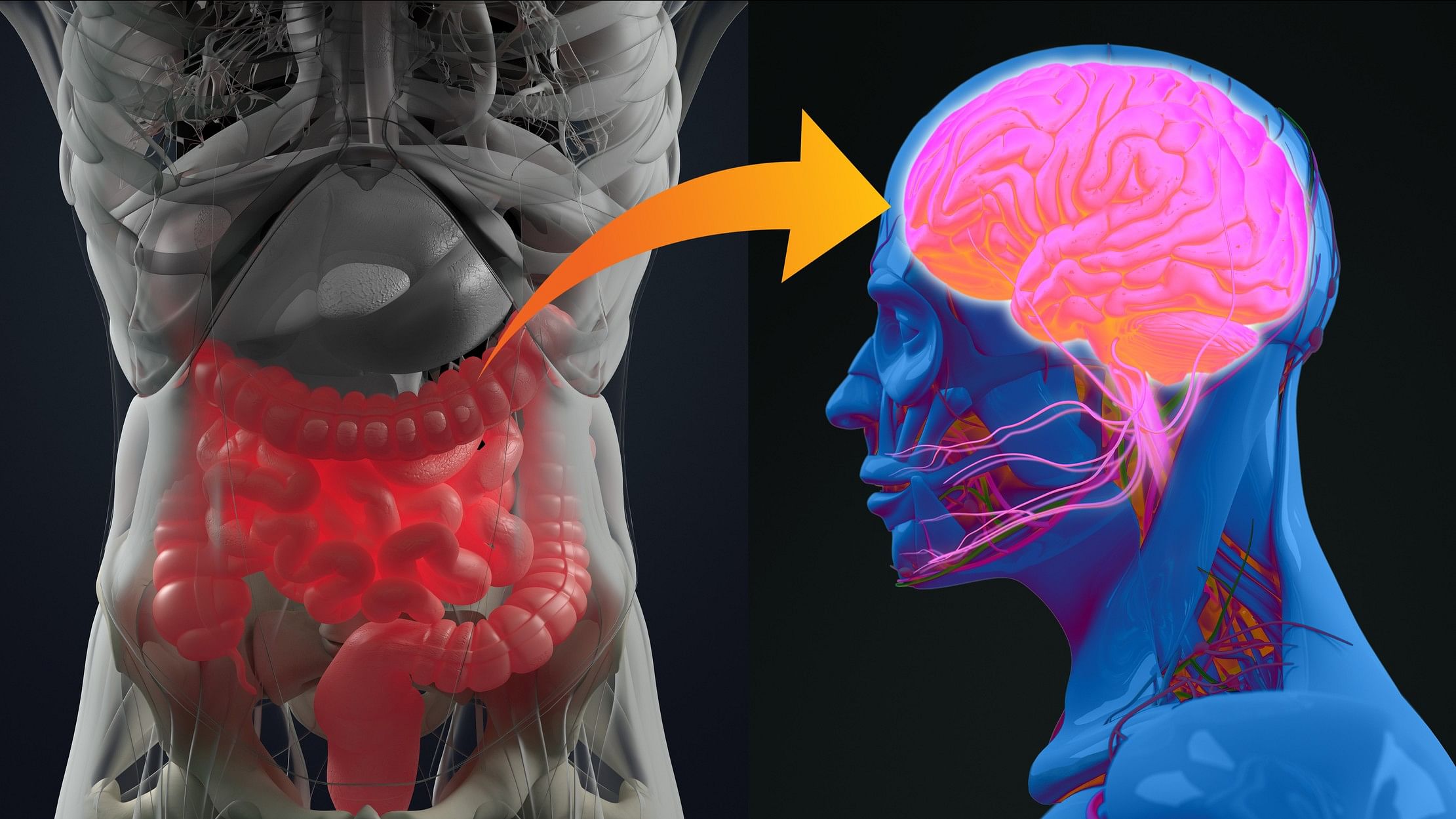
Representative image of the gut-brain axis.
Credit: iStock Photo
A microorganism, or a microbe, is an organism of microscopic size that either exists in a single-cell form or as a colony of cells. A vast majority of microbes are harmless or, as we shall see, beneficial to us. But ask Google the question “What is a microbe?” and this is the definition that pops up — “a microorganism, especially a bacterium causing disease or fermentation”.
This definition merely reflects humankind’s bias against this diverse and complex set of organisms. It’s likely that when you first read the word ‘microbe’, you had a mental image of these nasty, invisible creatures that are responsible for some of the most infamous diseases known to us. This image was, in all likelihood, accompanied by a feeling of fear or disgust. It is likely that the pandemic has compounded these feelings.
Microorganisms have existed on our planet for at least the last 3.5 billion years. To put that in perspective, our human ancestors have been around for a mere 6 million years, and modern humans only came into the picture 200,000 years ago. Science writer Ed Yong has a beautiful analogy that helps me wrap my head around these numbers: “Imagine the 4.54 billion-year history of the planet compressed into a single year. People have been around for only 30 minutes at most. Life emerged in March. It was all single-celled until October. Mammals and flowers didn’t emerge until early December, and dinosaurs ruled until the evening of December 26, when an asteroid wiped them out.”
While our microbial brethren have been around for aeons on every surface of our planet — with the exception of extreme environments like lava — it was only in the late 17th century that they came to be discovered by humans, when a Dutch cloth merchant happened to develop an interest in lens-making. At his ‘draper’ shop, Antonie van Leeuwenhoek needed a better device than the magnifying lenses of his time to inspect the quality of his threads and developed single-lens microscopes to do so. He turned his microscopes to examine pond water, and, lo and behold, it was teeming with “animalcules”!
Spotlight on good microbes
While Van Leeuwenhoek’s animalcules have now come to be known as microorganisms, his contributions to the establishment of the field of microbiology as a scientific discipline earned him the title ‘Father of Microbiology’. For ages, these fascinating organisms had remained hidden from humankind, like characters from the world of Harry Potter concealed beneath an invisibility cloak. But now the cloak had been cast off, unveiling their existence to all.
In the centuries that followed Leeuwenhoek’s discovery, microbes like bacteria came to be linked to deadly diseases like the plague, tuberculosis and cholera. The scientific world turned its focus on developing interventions against these deadly creatures, including antibiotics and vaccines, saving countless lives.
It is only in recent decades, however, that scientists have turned their attention to beneficial microorganisms. Scientists researching the ‘gut microbiome’ or the microorganisms that live in our digestive tracts have found that in its healthy state, this microbiome supports functions as wide-ranging as nutrient absorption, the regulation of immune responses, and the prevention of conditions like inflammatory bowel disease and allergies, to name a few.
Scientists like Justin Sonnenburg, a professor of microbiology and immunology at Stanford University, have suggested that over-sanitisation and overuse of antibiotics can reduce the diversity of our gut microbiome, which could have negative health consequences. Like a frustrated toddler painting over their entire picture with black paint instead of fixing a small error, humans have been sanitising or antibiotic-ing into oblivion entire microbial ecosystems, rather than targeting the harmful few.
Encouragingly, however, the gut microbiome seems to be quite malleable. It seems to respond to environmental cues like diet, antibiotic use, as well as exposure to environmental microbes. I am not going to get into these aspects or give you any specific dietary recommendations to improve your gut microbiome diversity, given that these are both out of the scope of this article as well as my areas of interest and expertise. There are resources out there, but I must caution the reader to proceed with extreme scepticism — the field is in its infancy and many of these ‘recommendations’ are unscientific and riddled with inconsistencies and exaggerated claims.
What’s the connection?
I mentioned earlier that our gut microbiome can help support our digestive and immune systems. But what if I now told you it has an impact on our brains as well? Gut microbes have been shown to influence neurological functions, and these links are referred to as the ‘gut-brain axis’. We all have an intuitive understanding of how our brains influence our gut — we’ve experienced the butterflies in our stomach when nervous, and have lost our appetites when sad. And we’ve also experienced the opposite, our gut affecting our brains and behaviour — think of the last time you were low on food. It’s likely this affected your mood and made you “hangry”, (hunger+anger) as the kids these days like to say.
Microbes can influence our brains and behaviour too. It’s strange to think that a microscopic organism could display such complexity, but examples from the natural world abound. Infection of rodents by the brain parasite Toxoplasma gondii appears to make them more exploratory and less fearful of predators like cats. This increases the likelihood of the parasite being transmitted to its definitive host, the cat, where it can reproduce. Some researchers have suggested that what underlies these behavioural changes in rodents is an immune response provoked by the T.gondii cysts in the brain.
What is interesting, said the researchers who conducted this study in an interview with Science magazine, is that these parasites have found a “sweet spot” — they invade the brain just enough to provoke this immune response that makes the rodents less fearful of predators, but not enough that the rodent dies right away without transmitting them to their primary feline hosts.
The rabies virus is also known to attack the brain, causing behavioural changes like aggression that increase the chances of the virus being transmitted via a bite to another host.
Switching gears back to our resident gut microbes, some of these bacteria have also been shown to influence social behaviour, anxiety and the stress response in humans and animals. Scientists do not yet fully understand the mechanisms by which the gut microbes impact our brain and behaviour, but there appear to be many possible conduits between these systems. For one, the microbes in the intestine can prompt the intestine to release hormones and other similar chemicals. These enter the blood flow and can affect processes controlled by the brain. Microbes living in the gut also produce neurotransmitters or brain-signalling chemicals.
Some bacterial strains have been shown in animal studies to hijack the signalling of the vagus nerve, which acts as a communication line between the gut and the brain, to alter behaviour. To cite just one example, scientists administered a subclinical dose of the diarrhoea-causing pathogen Campylobacter jejuni to mice and found that it caused increased anxiety-related behaviour, possibly by activation of the sensory neurons of the vagus nerve of the mice.
Is evidence piling up?
Some of the best evidence that microbes can influence animal brains and behaviour comes from the study of germ-free rodents, which are raised in laboratory conditions to prevent any microbe interactions since birth. These animals completely lack a microbiome. The effects of this have been found to be wide-ranging — these animals not only show alterations in gastric functions and immune functions, but they also show changes in the structure and function of the brain.
Scientists have found in these germ-free animals changes in the levels of brain chemicals called neurotransmitters, alterations in the production of new neurons, as well as structural changes to neurons in specific regions of the brain that control functions like fear and decision-making. Unsurprisingly, these germ-free rodents display anxiety-related and depressive-like symptoms, as well as alterations in social behaviours and cognition. There are also studies in the animal world that provide more direct links between the gut microbiome and the brain. Scientists have found that perturbing the gut microbiome of mice causes changes in their exploratory behaviour and alterations in the level of brain chemicals. Reminiscent of a plot from a sci-fi movie, there are studies that have also found that transferring the gut bacteria from an ‘adventurous’ mouse type to a more timid one causes the timid mouse to act more adventurously!
In humans, several studies have found that patients with brain-related disorders ranging from schizophrenia, major depression, Parkinson’s disease and autism spectrum disorder show drastic changes in their microbiome compared with controls. Of course, merely the fact that a correlation between changes in the gut microbiome and these disorders exists does not indicate that these perturbations are a cause of the disorder.
Interestingly, when gut microbes from patients with these disorders are transferred to germ-free mice, some of these symptoms start showing up in the mice as well. For instance, germ-free mice that were transplanted with the gut microbes of human patients with depression showed increased levels of anxiety and depressive behaviour. Similar findings have also been shown in Parkinson’s disease.
A long way to go
While these findings may be fascinating, there are several caveats to keep in mind while interpreting these studies on the connection between the gut microbiome and the brain. Most of these studies have been conducted on animals, and many of the human studies are correlational and suffer from low sample sizes. So while they may hint at a tantalising connection between some strains of the bacteria in our gut and the brain and/or behaviour, it is still early days to draw any significant conclusions about the causes of complex brain disorders from these.
If this article has left you with more questions than answers, that is thanks to the nature of the field and these complex microorganisms themselves. Scientists are only beginning to scratch the surface of understanding the world of these organisms and their relationship with us. As Ed Yong says: “We still have a lot to learn. But it’s worth making the journey because these are things that live in us, that live on us. They shape us. They influence us. We don’t really truly understand ourselves if we do not understand our relationships with them.”
The author is a neuroscience PhD turned science writer who is fascinated by the workings of the brain and how we can ‘rewire’ it to our advantage.
The Mind’s Eye is a bi-monthly column that explores neuroscience in everyday life.